Lab Startup Guide
The Office for Research has created this lab startup guide to help faculty new to VP&S orient themselves with the different offices responsible for research administration.
We hope that this guide will serve as an outline for the wet lab startup process and help new faculty begin their research as soon as possible.
This guide is intended to be an overview, and we encourage you to reach out directly to the offices listed below. They will provide you with information specific to your research, and guide you through the startup process. A complete guide to University research policies and handbooks is provided by the Office of the Executive Vice President for Research
If you have any questions about starting lab operations, please do not hesitate to contact your Department Administrator or any of the administration offices. Alternatively, you can always ask the Office for Research for assistance.
Timeline
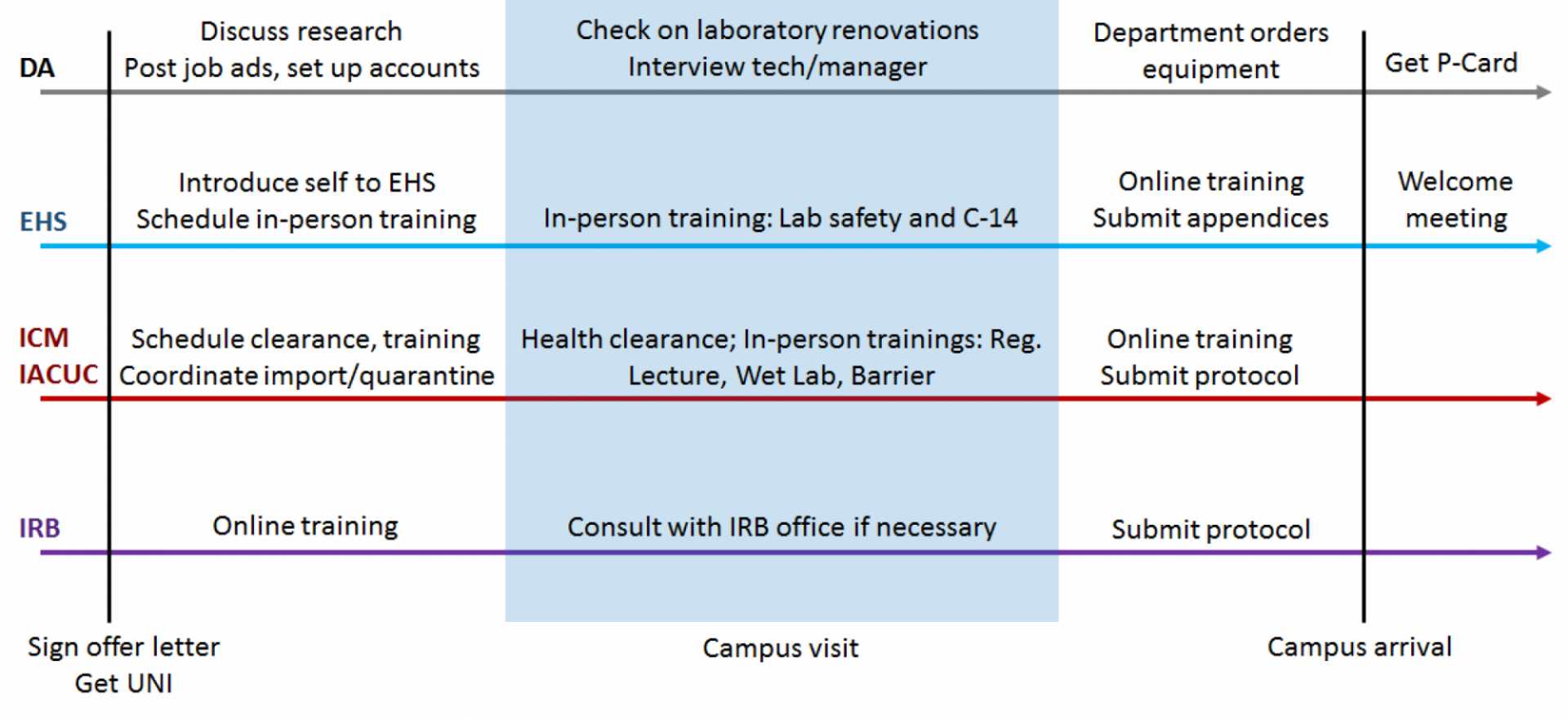
Your Department Administrator (DA) is your central contact for administrative issues, and has the ultimate authority to add you to systems, set up accounts, etc.
Early in the transition process
You should discuss all of your research requirements with your DA. The Department should then contact University offices to ensure that the required resources are allocated to you. Important items to discuss are:
-
Required space for your lab and offices, and any specialized equipment/resources needed
-
Planned animal research
-
Planned human subjects research, and whether the Department has special procedures for protocol oversight
-
Mechanisms of IT support
-
Whether technicians, students, or postdocs will work in the lab. The DA should explain the differences in cost for each of these positions.
-
If necessary, ask the DA to help prepare job postings for required staff. Ideally, a technician or manager will start at the same time as you arrive to help with the setup process.
As you get closer to your arrival on campus
It is important to discuss a business plan with your DA.
If you are moving into a newly renovated space, occasionally follow-up to see if construction will be completed on time. Having to move into a temporary space should be avoided.
Ask your Department to set up your funds account, get quotes, and place orders for vital pieces of laboratory equipment (biosafety hoods, incubators, freezers, etc.).
-
We have also set up an equipment exchange service, where you may be able to find laboratory equipment previously used by VP&S investigators that meets some of your needs
Shortly after you sign your offer letter, you should receive an email from HR containing your University Network ID (UNI). Your UNI is required to interact with most University systems, including online training and email systems.
Once you have your UNI and are officially appointed at Columbia University Irving Medical Center, you can obtain your Columbia University ID card (CUID) from the ID Office during a planned visit or upon your arrival. A CUID is required for entry to CUIMC buildings and should be carried at all times while on campus.
CUIMC uses an Exchange-based system for email that is compliant with HIPAA guidelines. This system is managed by CUIMC IT (note that CUIMC IT is distinct from CUIT), and can be set up once you have your UNI.
Environmental Health and Safety ensures that laboratories at CUIMC are operated in a safe manner, and deals with laboratory safety trainings, hazardous waste disposal, and biosafety issues. Radiation Safety is responsible for approval and monitoring of work involving radioactive materials and should be contacted if you plan to use such materials in your research.
You should set up a "welcome meeting" with EHS to discuss the steps involved in setting up your wet lab space. If a meeting was not set up for you by your Department, you can contact Chris Pitoscia.
At a minimum, wet lab researchers must complete two training courses in person:
-
Laboratory Safety, Chemical Hygiene and Hazardous Waste Management Training (held once a month)
-
Certificate of Fitness for Supervision of Chemical Laboratories (C-14; held every ~2 weeks)
Because these trainings are only offered on fixed dates, you should attend these training sessions during a visit to CUIMC or as soon as possible after your arrival. Additional in-person and online training may be required for your research. Schedules for all training courses are posted on the EHS website.
Animal research at CUIMC is primarily handled by two offices. The Institute of Comparative Medicine (ICM) handles issues related to physical facilities and veterinary services required for animal care. The Institutional Animal Care and Use Committee (IACUC) is responsible for compliance and approval of protocols necessary to perform animal research.
Importing animals
If you will be importing animals to CUIMC from another institution, the animals must be quarantined off-site before being transferred to Columbia. This takes at least 4 weeks, and can take longer if any health issues are detected. You will not be allowed to conduct animals studies until your IACUC protocol is approved (see below). If your IACUC protocol is not approved, animals may remain in off-site quarantine or be transferred to CUIMC and held under a holding protocol at cost to the investigator. Therefore, coordinate with ICM as soon as possible to ensure quarantine is completed on time and your IACUC protocol is approved for you to begin laboratory operations.
In-person clearance and training
Animal protocol approval and access to the animal facilities requires health clearance and a combination of in-person and online trainings. If possible, schedule these trainings to occur during visits to CUIMC or as soon as possible after your arrival to expedite protocol approval and facility access. Training/clearance links are on the IACUC and ICM websites.
Protocol approval
Protocol submission is performed via RASCAL, and consultations to assist with the process are available. Approval of Category B/C protocols can take approximately 4 weeks. Category D/E protocols require additional time and planning. D/E protocols require veterinary pre-review and submission to a full IACUC committee meeting.
The Human Research Protection Office (HRPO) handles submission and maintenance of Institutional Review Board (IRB) protocols.
IRB protocols are submitted via RASCAL. The HRPO maintains tutorials, tips, and example consent forms to assist in protocol generation.
All participants on an IRB protocol are required to complete human subjects protection training (via RASCAL).
A consultation service is available to assist in the IRB protocol submission and review process.
If you are transferring an IRB protocol from another institution, special considerations may apply. Contact the IRB Office for guidance.
Although purchase orders are required for certain items and services, it is easiest to purchase laboratory supplies and equipment using a P-card. Your Department Administrator, or another financial administrator in your department, can help you with the setup process.
While you are setting up your P-card, you can still use the purchase order system or have another member of your department who has a P-card transact your orders for you.
For most internal Columbia services (i.e. Facilities requests, use of shared resources through iLab, etc.), you will need a chartstring that is unique to each of your funding sources. This can be obtained from your DA/financial administrator.
Once your grants and other awards have been set up at Columbia and you have gone through the trainings required for ARC access, you can use the MyGrants website to view spending on your grants and make financial projections.
Ideally, you will have someone start at the same time as you arrive to help you set up your lab and start your research operations. However, it is important that you discuss this process with your Department Administrator or your department's HR manager. Columbia has several hiring policies that must be followed, and there are often technicalities regarding visas for postdoctoral scientists that will need to be addressed before you make an offer.
If you will be transferring grants from another institution to Columbia, the Sponsored Projects Administration (SPA) will assist you. SPA will also coordinate with you and your previous institution to draft any Material Transfer Agreements (MTAs) that are required for you to continue your research. Specific Project Officers and Financial Analysts are assigned to each school, department, institute, and center at the University to assist with sponsored projects. Contact the SPA office to determine who will assist you.
If you are bringing major equipment from another institution, your department's administrators should contact the other institution to obtain an inventory of major equipment to be transferred, and send this list to Capital Asset Accounting to ensure there is a record of your items.
Once your grants and other awards have been set up at Columbia and you have gone through the trainings required for ARC access (see the Purchasing section above), you can use the MyGrants website to make financial projections.
The VP&S Office for Research and university-wide Office of the Executive Vice President for Research offer a number of resources to help you identify and apply for internal and external grants:
- You should determine who in your Department provides pre-award support to help you prepare and submit your application. Several departments utilize the VP&S Grants Management Services to provide this support.
- We have assembled a list of funding opportunities
- Our Grant Toolbox contains checklists and primers for application assembly, a facilities and resources bank, and other tools to help you submit your proposal
- A number of grant courses can assist if this is the first time you are submitting a grant
- The Office for Research can assist with large and complex research grants